Health Canada warns of Illegal eye drops, skin care care products being sold in Metro Vancouver

Metro Vancouver residents are being warned about illegal eye drops, as well as skin care products, that are currently being sold at two different locations in the region.
In a statement, Health Canada warns that the products are “unauthorized and may pose serious health risks.”
See also
- Nova Scotia introduces presumed consent for organ donation
- The number of global measles cases is already 300% higher than this time last year
- More YVR passengers potentially exposed to measles: health officials
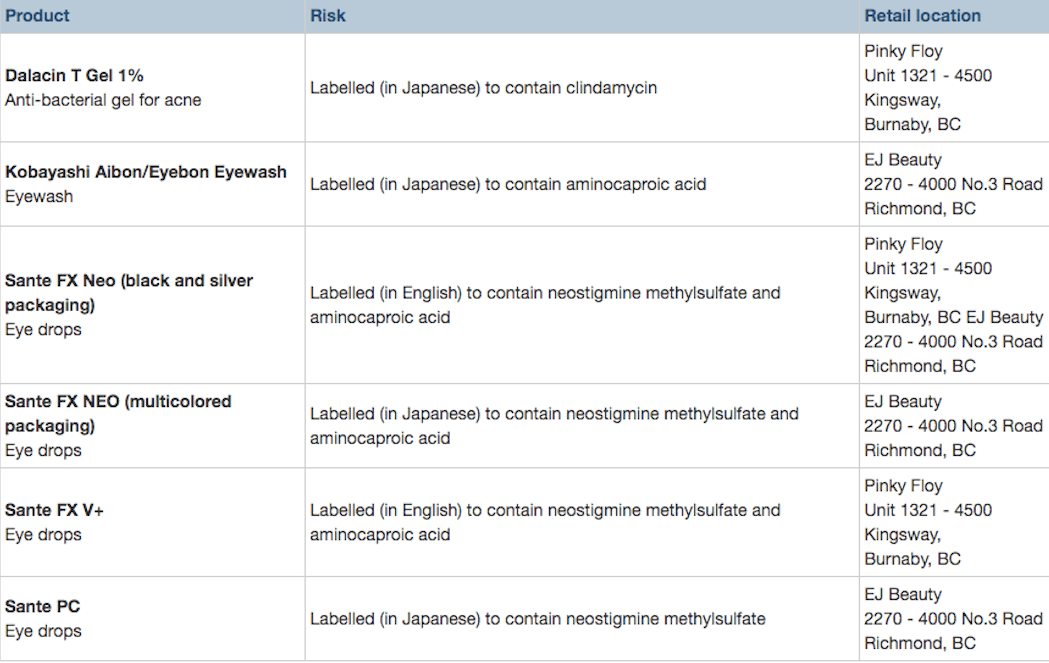
According to the federal agency, the products – being sold at Pinky Floy in Burnaby, as well as EJ Beauty in Richmond – contain prescription drugs.
“Prescription drugs should be taken only under the advice and supervision of a healthcare professional because they are used to treat specific diseases and may cause serious side effects,” Health Canada warns.
In addition, “the unauthorized health products have not been approved by Health Canada, which means that they have not been assessed for safety, effectiveness and quality and may pose serious health risks.”
Health Canada also notes that some of the product’s labelling is in Japanese, and as a result, “information about ingredients, usage, dosage and side effects may not be understood by all consumers.”
Health Canada
What to do
Anyone in possession of the affected products are advised to do the following:
- Stop using these products. Consult your health care professional if you have used any of these products and have health concerns.
- Read product labels to verify that health products have been authorized for sale by Health Canada. Authorized health products have an eight-digit Drug Identification Number (DIN), Natural Product Number (NPN) or Homeopathic Drug Number (DIN-HM). You can also check whether products have been authorized for sale by searching Health Canada’s Drug Product Database and Natural Health Product Database.
- Report any health product-related adverse reactions or complaints to Health Canada.
Background
Aminocaproic acid: Prescription drug ingredient used to decrease bleeding in various clinical situations. Exposure to aminocaproic acid in the eye may affect the eye itself, and the acid may be absorbed through the tear ducts into the blood. Side effects may include watery eyes, vision changes, headache, dizziness, nausea, muscle weakness and skin rash.
Clindamycin: In topical format is a prescription antibiotic approved in Canada to treat bacterial infections, including those associated with acne. The product should not be used in individuals with a history of ulcerative colitis (inflamed bowel) or a history of inflamed bowel associated with antibiotic use. Side effects may include dry or scaly skin, peeling skin, a stinging or burning feeling on the skin, eye pain, itching, hives, redness and gastrointestinal symptoms.
Neostigmine methylsulfate: There are no approved eye drops containing neostigmine methylsulfate on the Canadian market. These medications are no longer widely used because of the significant number of potential eye-related side effects, including blurred distance vision, frontal headaches, twitching lids, red eyes, cataracts, allergic reactions, iris cysts, retinal detachment, and the potential for causing a specific type of glaucoma attack.

